
| 作者及发表 年份 | 试验编号 | 国家 | 研究类型 | 患者分期 | 治疗 类型 | 治疗方案 | 人数 | 主要结局 | 次要结局 |
|---|---|---|---|---|---|---|---|---|---|
| Fernandez-Martos等2010[ | GCR-3 | 西班牙 | Phase Ⅱ RCT | cT3-4N0-2M0 | B | XELOX+RT | 52 | ① | ②④⑦ |
| C | XELOX+CRT | 56 | |||||||
| Borg等 2014[ | INOVA | 法国 | Phase Ⅱ RCT | T3 | A | Be+5-FU+RT | 44 | ①② | ④⑦ |
| C | Be+FOLFOX+CRT | 46 | |||||||
| Maréchal等 2012[ | 美国 | Phase Ⅱ RCT | T2-4/N+ | A | 5-FU+RT | 29 | ②③ | ①⑦ | |
| C | FOLFOX+CRT | 28 | |||||||
| Cho等 2016[ | 韩国 | Phase Ⅱ RCT | cT3-4N0 cT1-4N1-2 | B | XELOX+RT | 20 | ④ | ⑨⑧⑦ | |
| C | XELOX+CRT | 18 | |||||||
| Conroy等 2021[ | PRODIGE 23 | 法国 | Phase Ⅲ RCT | cT3-4 | A | CAP+RT | 230 | ⑤ | ⑧④⑦ |
| D | FOLFIRINOX+CRT | 231 | |||||||
| Calvo等 2014[ | 西班牙 | Cohort study | cT3-4N0-2M0 | A | 5-FU+RT | 128 | ② | ⑦ | |
| C | FOLFOX+CRT | 207 | |||||||
| Cercek等 2018[ | 美国 | Retrospective cohort study | cT3-4N0-2M0 | A | 5-FU or CAP+RT | 296 | ① | ||
| C | FOLFOX or XELOX +CRT | 235 | |||||||
| Garcia-Aguilar等 2015[ | 美国 | Phase Ⅱ RCT | cT3-4N0 cT1-4N1-2 | A | 5-FU+RT | 60 | ① | ⑩⑪ | |
| E | CRT+mFOLOFOX6 | 67 | |||||||
| Kim等 2018[ | 韩国 | Phase Ⅱ RCT | cT3-4N0-2M0 | A | CAP+RT | 52 | ② | ①⑦ | |
| E | CRT+XELOX | 44 | |||||||
| Wang等 2019[ | FDRT-002 | 中国 | Phase Ⅱ RCT | cT3-4N0-2M0 | B | XELOX+RT | 53 | ① | ⑤⑧⑦ |
| E | CRT+XELOX | 57 | |||||||
| Zhai等 2020[ | 中国 | Cohort study | cT3-4N0-2M0 | A | CAP+RT | 78 | ① | ②⑦ | |
| E | CRT+XELOX | 110 | |||||||
| Moore等 2017[ | WAIT trial | 澳大利亚 新西兰 | Phase Ⅱ RCT | T3, T4 | A | 5-FU+RT | 24 | ① | ④ |
| E | CRT+5-FU | 25 | |||||||
| Bujko等 2016[ | 波兰 | Phase Ⅲ RCT | cT3 or cT4 | A | 5-FU+RT | 254 | ④ | ①⑧⑤ ⑦ | |
| F | 5×5Gy+FOLFOX4 | 261 | |||||||
| Bujko等 2013[ | 波兰 | Phase Ⅲ RCT | cT3 or cT4 | B | CAPOX+RT | 48 | ④ | ①②⑦ | |
| F | 5×5Gy+FOLFOX4 | 49 | |||||||
| Maxime等 2020[ | RAPIDO trial | 多中心 | Phase Ⅲ RCT | T2–4 and/or N+ | A | CAP+RT | 450 | ⑥ | ①④⑧ ⑦ |
| F | 5×5Gy+XELOX | 462 | |||||||
| Markovina等 2017[ | 美国 | Phase Ⅱ RCT | cT3-4N0-2M0 | A | 5-FU or CAP+RT | 69 | ① | ②⑧⑤⑦ | |
| F | 5×5Gy+FOLFOX | 69 | |||||||
| Aghili等 2020[ | 伊朗 | Phase Ⅱ RCT | cT3-4N0-2M0 | A | CAP+RT | 31 | ⑦ | ①②④ | |
| F | 5×5Gy+ XELOX | 35 | |||||||
| Gérard等 2012[ | ACCORD 12 | 美国 | Phase Ⅲ RCT | cT2-4N0-2M0 | A | CAP+RT | 299 | ⑧ ⑤ | ⑦④ |
| B | XELOX+RT | 299 | |||||||
| O’Connell等 2014[ | NSABP R04 | 美国 | Phase Ⅲ RCT | cT3/T4 or Tx N+M0 | A | CAP+RT | 636 | ① | ⑦② |
| B | XELOX+RT | 640 | |||||||
| Haddad等2015[ | 伊朗 | Phase Ⅱ RCT | T3,T4 or N+ | A | CAP+RT | 31 | ① | ②⑦ | |
| B | XELOX+RT | 32 | |||||||
| Schmoll等2020[ | PETACC 6 | 美国 | Phase Ⅲ RCT | T3,T4 or N+ | A | CAP+RT | 523 | ⑤ | ①④② |
| B | XELOX+RT | 497 | |||||||
| Valentini等 2019[ | INTERACT | 意大利 | Phase Ⅲ RCT | cT2N0–2M0 cT3N0–2M0 | A | CAP+RT | 267 | ① | ⑧⑤⑦ ② |
| B | XELOX+RT | 245 | |||||||
| Fokas等 2019[ | CAO/ARO/AIO-12 | 德国 | Phase Ⅱ RCT | cT3-4N0-2M0 | C | FOLFOX+CRT | 156 | ① | ⑦②④ |
| E | CRT+FOLFOX | 156 |
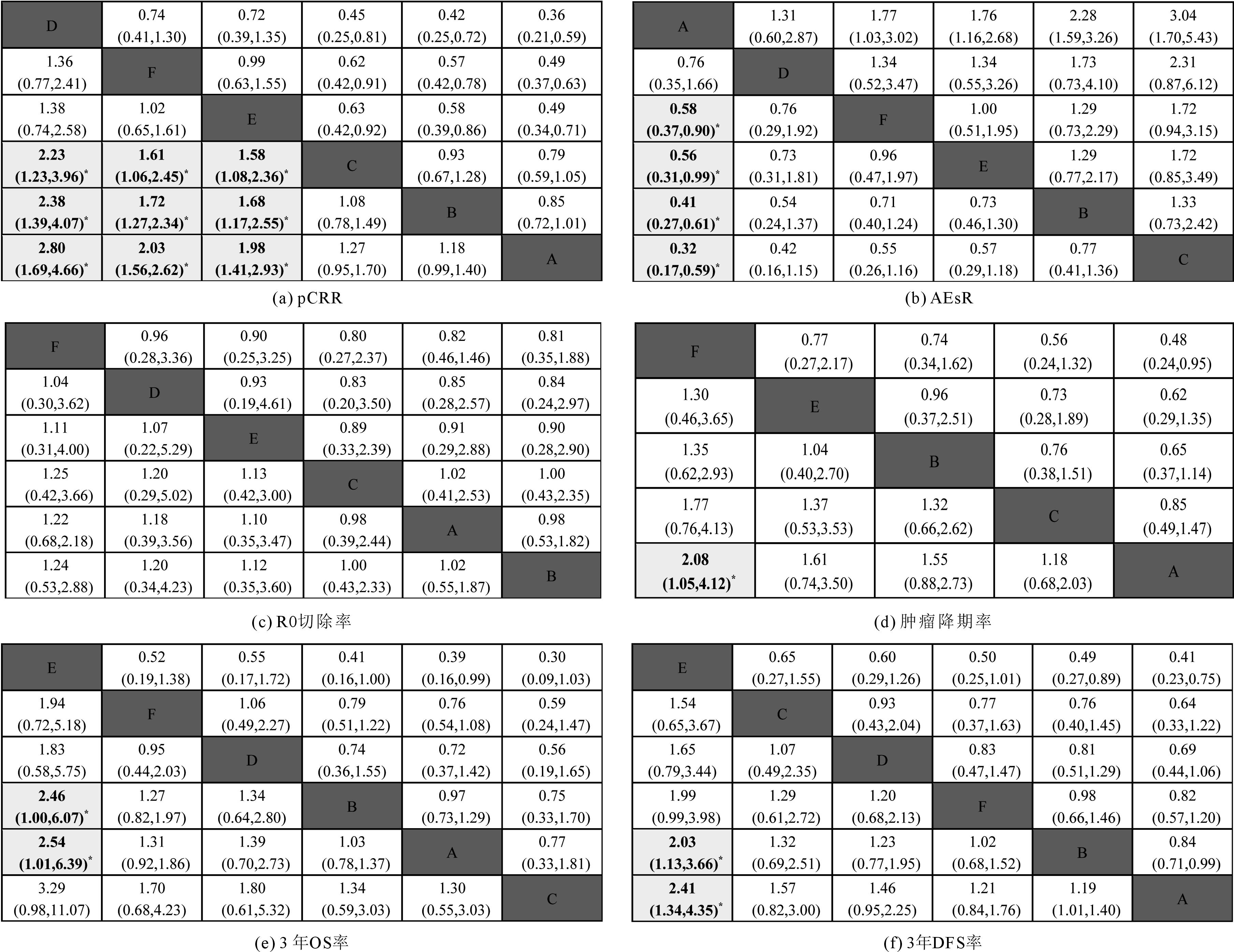 Fig 4 Odds ratio (OR) and 95%CI of all regimens from network Meta-analysis for six indicators 注(note):中间深色板块将联赛表分为上下两部分,OR(odds ratio) 值代表列与行两方案的比值比[different interventions in the middle blocle divded the graph into upper and lower triangle;the OR (odds ratio) value represents the odds ratio of the two prototocols in the column and row];95%CI 不包括1.00代表两方案有显著性差异,用灰色方块表示,且加粗标有星号(95%CI excluding 1.00 means there is a significant difference between the two protocols and is represented by a gray square with a bold asterisk)。
Fig 4 Odds ratio (OR) and 95%CI of all regimens from network Meta-analysis for six indicators 注(note):中间深色板块将联赛表分为上下两部分,OR(odds ratio) 值代表列与行两方案的比值比[different interventions in the middle blocle divded the graph into upper and lower triangle;the OR (odds ratio) value represents the odds ratio of the two prototocols in the column and row];95%CI 不包括1.00代表两方案有显著性差异,用灰色方块表示,且加粗标有星号(95%CI excluding 1.00 means there is a significant difference between the two protocols and is represented by a gray square with a bold asterisk)。